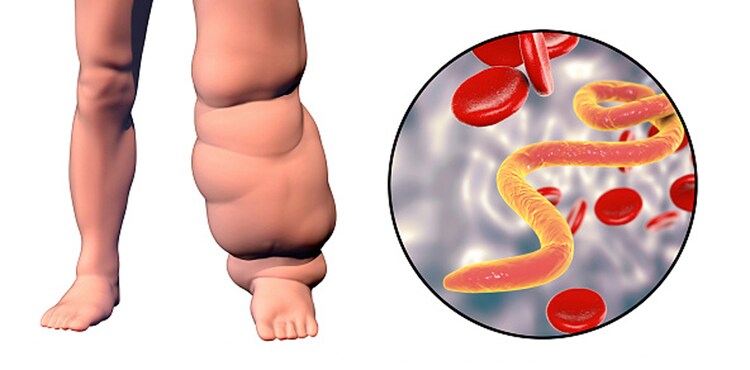
The term “lymphatic filariasis” covers infection with three closely related nematode worms – W. bancrofti, B. malayi and B. timori. All three infections are transmitted to humans through the bite of infectious mosquitoes. All three parasites have basically similar life cycles in man-adult worms living in lymphatic vessels whilst their offspring, the microfilariae circulate in peripheral blood and are available to infect mosquito vectors when they come to feed.
About 95 per cent of cases of lymphatic filariasis are caused by infection with W. bancrofti; other related parasites that infect humans are Brugia malayi in South-East Asia and B. timori in Indonesia. The formal goal of the global lymphatic filariasis programme is to eliminate the disease “as a public health problem” and 2020 is the informal target date for interrupting transmission.
Agent factors:
There are at least 8 species of filarial parasites that are specific to man. The first three worms are responsible for lymphatic filariasis; and the rest for “non-lymphatic filariasis”.
| Organism | Vectors | Disease produced |
| Wuchereria bancrofti | Culex mosquito | lymphatic filariasis |
| Brugia malayi | Mansonia mosquito | lymphatic filariasis |
| Brugia timori | Anopheles mosquito Mansonia mosquito | lymphatic filariasis |
| Onchocerca volvulus | Simulum flies | Subcutaneous; nodules; |
| Loa loa | Chrysops flies | Recurrent, transient subcufaneous swellings |
| T. perstans | Culicoides | Probably rarely any clinical illness |
| T. streptocerca | Culicoides | Probably rarely any clinical illness |
| Mansonella ozzardi | Culicoides | Probably rarely any clinical illness |
Periodicity:
The Mf of W bancrofti and B. malayi occurring in India display a nocturnal periodicity, i.e., they appear in large numbers at night and retreat from the blood stream during the day. This is a biological adaptation to the nocturnal biting habits of the vector mosquitoes. The maximum density of Mf in the blood is reported between 10 pm and 2 am. When the sleeping habits of the host are changed, a reversal of periodicity has been observed.
Life Cycle:
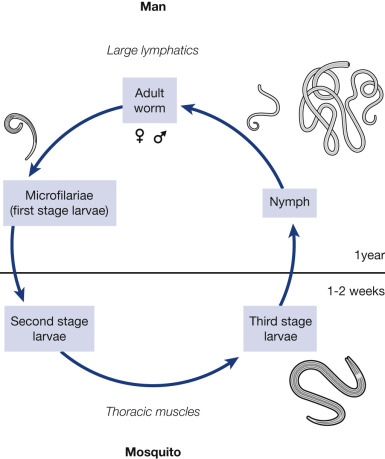
The mosquito cycle begins when the Mf are ingested by the vector mosquito during feeding. The following stages of development take place in the vector:
Exsheathing:
The larva emerges from the envelope in which it is enclosed within 1 to 2 hours after ingestion. This is called exsheathing and takes place in the stomach of the mosquito.
First stage larva:
After exsheathing, the larva is able to penetrate the stomach wall of ‘the mosquito which it does in 6 to 12
hours and migrate to the thoracic muscles where it grows and develops into a sausage-shaped (short, thick) form.
Second stage larva:
The larva moults and increases in length (long, thick form) with the development of an alimentary canal, but is relatively inactive.
Third stage larva :
There is a final moult to the third stage or infective larva (long, thin form), which may be in any part of the insect. It is highly active or motile. When it migrates to the proboscis of the mosquito, it is ready to be transmitted to a new host and the mosquito is considered infectious.
Host factors:
Man is a natural host.
AGE:
All ages are susceptible to infection. In endemic areas, filarial infection has even been found in infants under 6 months of age. Infection rates rise with age up to the age of 20-30 years and then level off. After a few years at this plateau level, Mf rates may decline in middle and old age. Filarial disease appears only in a
small percentage of infected individuals, commonly in the age group over 10 years, although there may be
exceptions.
SEX:
In most endemic areas the Mf rate is higher in men.
MIGRATION:
The movement of people from one place to another has led to the extension of filariasis into areas previously non-endemic.
IMMUNITY:
Man may develop resistance to infection only after many years of exposure. The immunological basis for this resistance is not known.
SOCIAL FACTORS:
Lymphatic filariasis is often associated with urbanization, industrialization, migration of people, illiteracy, poverty and poor sanitation.
Environmental factors:
CLIMATE:
Climate is an important factor in the epidemiology of filariasis. It influences the breeding of mosquitoes, their longevity and also determines the development of the parasite in the insect . vector. The maximum prevalence of Culex quinquefasciatus (previously known as C. fatigans) was observed when the temperature
was between 22 to 38 deg. C and optimum longevity when the relative humidity was 70 per cent.
DRAINAGE:
Lymphatic filariasis is associated with poor drainage. The vectors breed profusely in polluted water.
TOWN PLANNING:
Inadequate sewage disposal and lack of town planning have aggravated the problem of filariasis in India
by increasing the facilities for the breeding of C. quinquefasciatus (C. fatigans). The common breeding places are cesspools, soakage pits, ill-maintained drains, septic tanks, open ditches, burrow pits, etc.
Mode of transmission:
Filariasis is transmitted by the bite of infected vector mosquitoes. The parasite is deposited near the site of
puncture. It passes through the punctured skin or may penetrate the skin on its own and finally reach the lymphatic system. The dynamics of transmission depends upon the man-mosquito contact.
Incubation period:
The time interval between inoculation of infective larvae and the first appearance of detectable Mf is known as “prepatent period”. Direct information on the duration of the prepatent period is lacking. The time interval from invasion of infective larvae to the development of clinical manifestations is known as the “clinical incubation period”. This period, most commonly, is 8 to 16 months.
Clinical manifestations:
Only a small proportion of infected individuals exhibit, clinical signs. The disease manifestations can be divided into two distinct clinical types : (a) lymphatic filariasis caused by the parasite in the lymphatic system, and (b) occult filariasis caused by ah immune hyper-responsiveness of the human host
LYMPHATIC FILARIASIS:
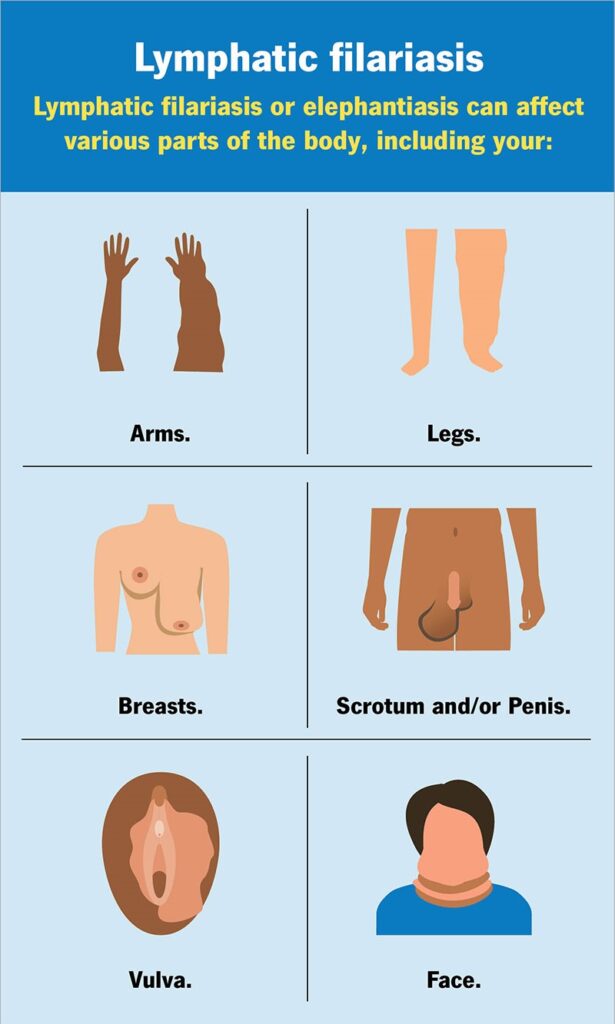
Asymptomatic amicrofilaraemia:
In all endemic areas a proportion of population does not show Mf or clinical manifestations of the disease although they have the same degree of exposure to infective larvae as those who become infected. With presently available diagnostic procedures it is not possible to determine whether persons in this group have detectable infections or whether they are free from infection.
Asymptomatic microfilaraemia:
A considerable proportion of people are asymptomatic, although their blood is positive for Mf. They may remain without any symptoms for months – in some instances for years. They are an important source of infection in the community. These carriers are usually detected by night blood examination.
Stage of acute manifestations:
In the first months and years there are recurrent episodes of acute inflammation in lymph glands and vessels. The clinical manifestations comprise filarial fever, lymphangitis, lymphadenitis, lymphoedema of the various parts of the body and of epididymo-orchitis in the male.
Stage of chronic obstructive lesions:
The chronic stage usually develops 10-15 years from the onset of the first acute attack. This phase is due to fibrosis and obstruction of lymphatic vessels causing permanent structural changes.
OCCULT FILARIASIS:
The term occult or cryptic filariasis refers to filarial infections in which the classical clinical manifestations are
not present and Mf are not found in the blood. It is assumed that occult filariasis is due to a hypersensitivity reaction to filarial antigens of Mf. The best known example is tropical pulmonary eosinophilia.
 Users Today : 8
Users Today : 8 Users Yesterday : 2
Users Yesterday : 2 Users Last 7 days : 20
Users Last 7 days : 20 Users Last 30 days : 71
Users Last 30 days : 71 Users This Month : 71
Users This Month : 71