
More industrial workers are exposed to lead than to any other toxic metal.
Lead is used widely in a variety of industries because of its properties :
- low boiling point
- mixes with other metals easily to form alloys
- easily oxidised
- anticorrosive.
All lead compounds are toxic – lead arsenate, lead oxide and lead carbonate are the most dangerous; lead sulphide is the least toxic.
INDUSTRIAL USES:
Over 200 industries are counted where lead is used – manufacture of storage batteries; glass manufacture; ship building; printing and potteries; rubber industry and several others.
NON-OCCUPATIONAL SOURCES:
The greatest source of environmental (non-occupational) lead is gasoline. Thousands of tons of lead every year is exhausted from automobiles. Lead is one of the few trace metals that is abundantly present in the environment Lead exposure may also occur through drinking water from lead pipes; chewing lead paint on window sills or toys in case of children.
MODE OF ABSORPTION:
Lead poisoning may occur in three ways:
INHALATION:
Most cases of industrial lead poisoning is due to inhalation of fumes and dust of lead or its compounds.
INGESTION:
Poisoning by ingestion is of less common occurrence. Small quantities of lead trapped in the upper respiratory tract may be ingested. Lead may also be ingested in food or drink through contaminated hands.
SKIN:
Absorption through skin occurs only in respect of the organic compounds of lead, especially tetraethyl lead. Inorganic compounds are not absorbed through the skin.
BODY STORES:
The body store of lead in the average adult population is about 150 to 400 mg and blood levels average about 25μg/100 ml. An increase to 70μg/100 ml blood is generally associated with clinical symptoms. Normal adults ingest about 0.2 to 0.3 mg of lead per day largely from food and beverages.
CLINICAL FEATURES:
The clinical picture of lead poisoning or plumbism is different in the inorganic and organic lead exposures. The toxic effects of inorganic lead exposure are abdominal colic, obstinate constipation, loss of appetite, blue-line on the gums, stippling of red cells, anemia, wrist drop and foot drop. The toxic effects of organic lead compounds are mostly on the central nervous system insomnia, headache, mental confusion, delirium.
DIAGNOSIS:
Diagnosis of lead poisoning is based on:
HISTORY:
a history of lead exposure
CLINICAL FEATURES:
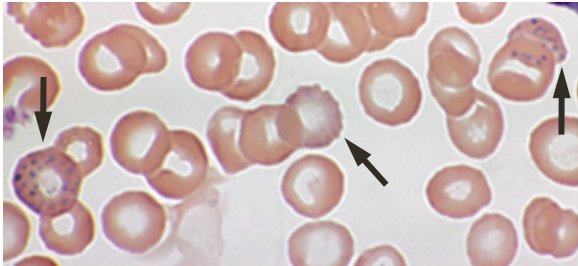
such as loss of appetite, intestinal colic, persistent headache, weakness, abdominal cramps and constipation, joint and muscular pains, blue line on gums, anemia.
LABORATORY TESTS:
- (a) Coproporphyrin in urine: Measurement of CPU is a useful screening test. In non-exposed persons, it is less than 150 microgram/liter.
(b) Amino levulinic acid in urine: If it exceeds 5 mg/ liter, it indicates clearly lead absorption. - (c) Lead in bloodand urine : Measurement of lead in blood or urine requires refined laboratory techniques. They provide quantitative indicators of exposure. Lead in urine of over 0.8 mg/litre (normal is 0.2 to 0.8 mg) indicates lead exposure and lead absorption. A blood level of 70μg/100 ml is associated with clinical symptoms.
- (d) Basophilic stipling of RBC : Is a sensitive parameter of the hematological response
PREVENTIVE MEASURES:

Substitution:
That is, where possible lead compounds should be substituted by less toxic materials.
Isolation:
All processes which give rise to harmful concentration of lead dust or fumes should be enclosed and segregated.
Local exhaust ventilation:
There should be adequate local exhaust ventilation system to remove fumes and dust promptly.
Personal protection:
Workers should be protected by approved respirators.
Good house-keeping:
Good house-keeping is essential where lead dust is present. Floors, benches, machines should be kept clean by wet sweeping.
Working atmosphere:
Lead concentration in the working atmosphere should be kept below 2.0 mg per 10 cu. meters of air, which is usually the permissible limit or threshold value.
Periodic examination of workers:
All workers must be given periodical medical examination. Laboratory determination of urinary lead, blood lead, red cell count, hemoglobin estimation and coproporphyrin test of urine should be done periodically. Estimation of basophilic stippling may also be done. An Expert Committee of the WHO states that in the case of exposure to lead, it is not only the average level of lead in the blood that is important, but also the number of subjects whose blood level exceeds a certain value.
Personal hygiene:
Handwashing before eating is an important measure of personal hygiene. There should be adequate washing facilities in industry. Prohibition on taking food in work places is essential.
Health education:
Workers should be educated on the risks involved and personal protection measures.
MANAGEMENT:
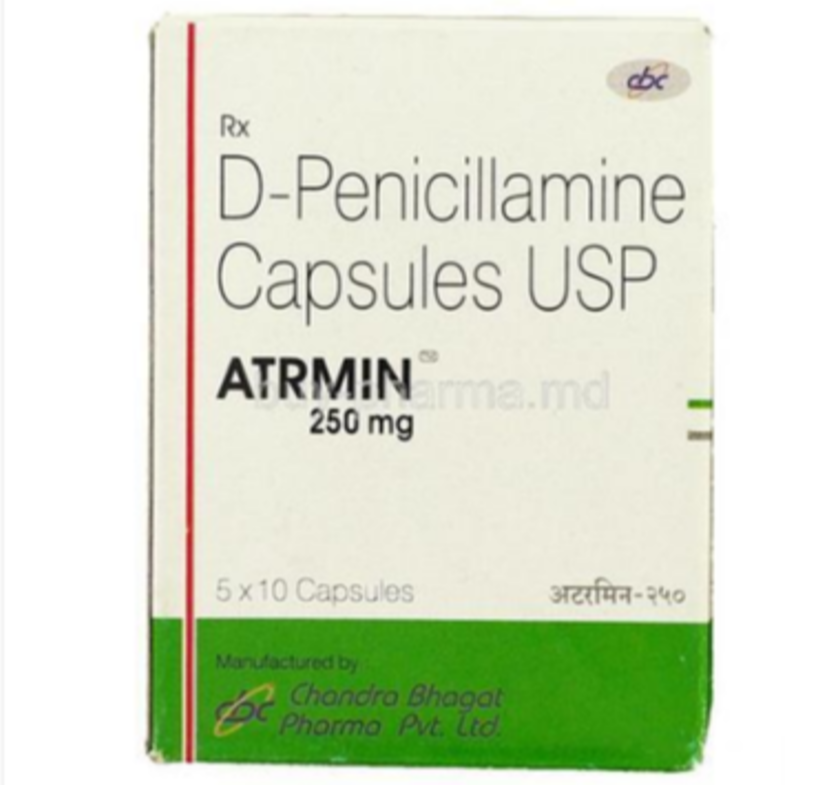
The major objectives in management of lead poisoning are the prevention of further absorption,
the removal of lead from soft tissues and prevention of recurrence. Early recognition of cases will help in removing· them from further exposure. A saline purge will remove unabsorbed lead from the gut. The use of d-penicillamine has been reported to be effective. Like Ca-EDTA, it is a chelating agent and works by promoting lead excretion in urine.
 Users Today : 1
Users Today : 1 Users Yesterday : 2
Users Yesterday : 2 Users Last 7 days : 16
Users Last 7 days : 16 Users Last 30 days : 102
Users Last 30 days : 102 Users This Month : 75
Users This Month : 75